Design for Deeper Learning
Collaborate with AI to design an engaging learning experience in minutes.
Try it out – design your own projectCollaborate with AI to design an engaging learning experience in minutes.
Try it out – design your own project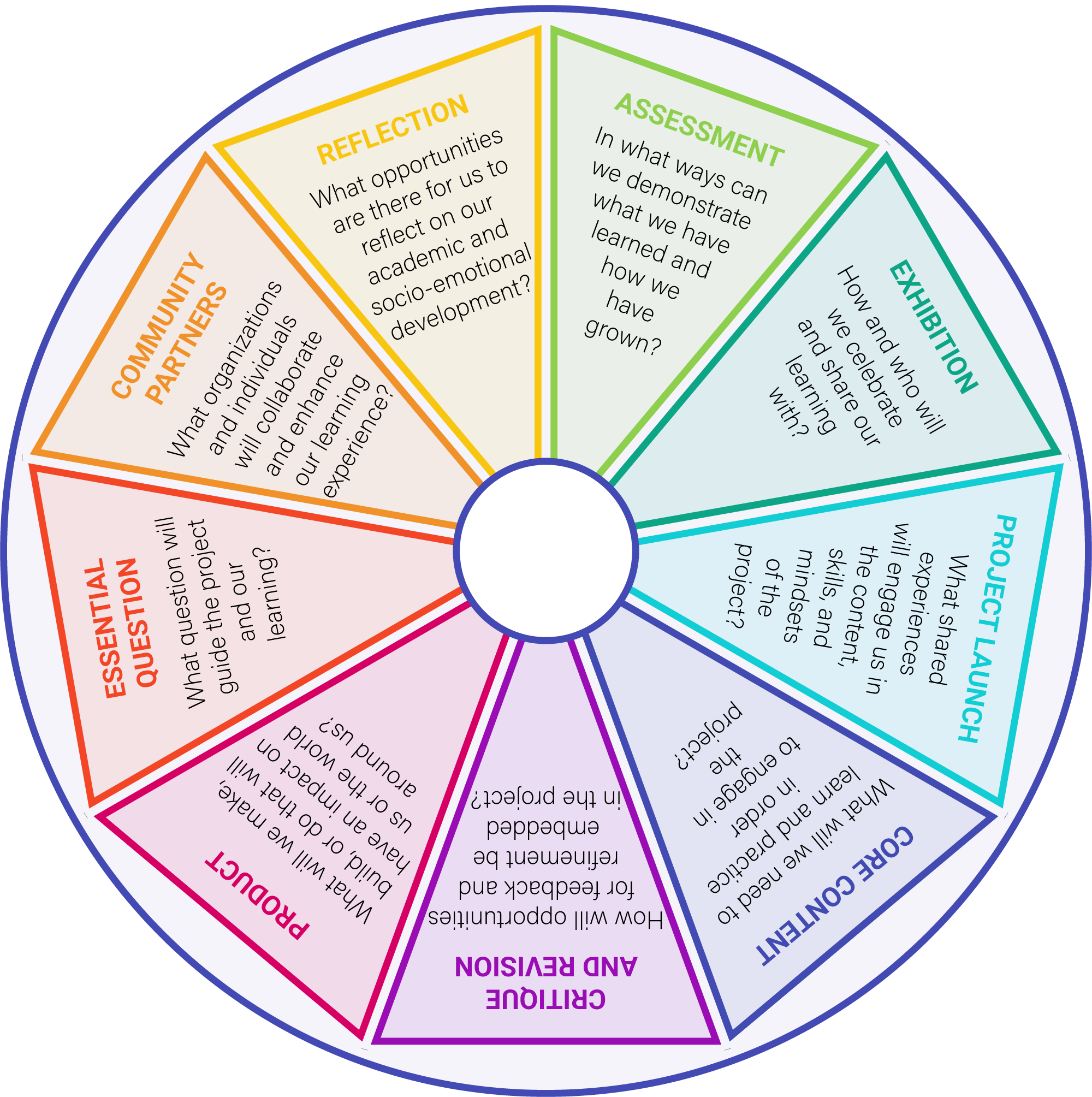

First Grade Trash PBL
Grade Level:

CPR Champions: Seniors saving lives.
Grade Level:

Tide Pools & Pokemon: Math Meets Marine Magic
Grade Level:

Planetary Parade: Crafting the Cosmic Scale
Grade Level:

Global Flavors Fiesta: Cultures on Display!
Grade Level:

Borderland Bonanza: Game of Geography & Economy!
Grade Level:

Mind Matters: Exploring the Brain's Role
Grade Level:

Walk Back the Cat: digging deeper into US history
Grade Level:
What if there was a tool to help us take our wild project ideas and create a scope and sequence? There is! Inkwire and the Professional Learning team at High Tech High’s Graduate School of Education designed an AI-assisted curriculum planning tool.
Powered by High Tech High's Kaleidoscope framework for project-based learning (PBL) design, this AI assistant helps educators – and learners! – integrate standards and curriculum requirements into a cycle of PBL Essentials.